Atmospheric chemistry
 |
| Meteorology |
|---|
| Climatology |
| Aeronomy |
| Glossaries |
| Part of a series on |
| Weather |
|---|
|
|
Atmospheric chemistry is a branch of atmospheric science that studies the chemistry of the Earth's atmosphere and that of other planets. This multidisciplinary approach of research draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and volcanology, climatology and other disciplines to understand both natural and human-induced changes in atmospheric composition. Key areas of research include the behavior of trace gasses, the formation of pollutants, and the role of aerosols and greenhouse gasses. Through a combination of observations, laboratory experiments, and computer modeling, atmospheric chemists investigate the causes and consequences of atmospheric changes.
The composition and chemistry of the Earth's atmosphere is important for several reasons, but primarily because of the interactions between the atmosphere and living organisms. Natural processes such as volcano emissions, lightning and bombardment by solar particles from corona changes the composition of the Earth's atmosphere. It has also been changed by human activity and some of these changes are harmful to human health, crops and ecosystems. Examples of problems addressed in atmospheric chemistry include acid rain, ozone depletion, photochemical smog, greenhouse gasses and global warming. Atmospheric chemists work to understand the causes of these problems. By developing a theoretical understanding, they can test potential solutions and evaluate the effects of changes in government policy.
Atmospheric composition
[edit]


| Average composition of dry atmosphere (mole fractions) | ||
|---|---|---|
| Gas | Dry air per NASA | Dry clean air near sea level (standard ISO 2533 - 1975) |
| Nitrogen, N2 | 78.084% | 78.084% |
| Oxygen, O2[2] | 20.946% | 20.946% |
| Minor constituents (mole fractions in ppm) | ||
| Argon, Ar | 9340 | 9340 |
| Carbon dioxide*[a], CO2 | 430 | 430 |
| Neon, Ne | 18.18 | 18.18 |
| Helium, He | 5.24 | 5.24 |
| Methane[a], CH4 | 1.9 | 1.9 |
| Krypton, Kr | 1.14 | 1.14 |
| Hydrogen, H2 | 0.53 | 0.53 |
| Nitrous oxide, N2O | 0.34 | |
| Xenon, Xe | 0.087 | |
| Nitrogen dioxide, NO2 | up to 0.02 | |
| Ozone*, O3, in summer | up to 0.07 | |
| Ozone*, O3, in winter | up to 0.02 | |
| Sulphur dioxide*, SO2 | up to 1 | |
| Iodine*, I2 | 0.01 | |
| Water | ||
| Water vapour* | Highly variable (about 0–3%); typically makes up about 1% | |
| Notes | ||
| The mean molecular mass of dry air is 28.97 g/mol. *The content of the gas may undergo significant variations from time to time or from place to place. [a]The concentration of CO2 and CH4 vary by season and location. | ||
Trace gas composition
[edit]Besides the major components listed above, the Earth's atmosphere contains many trace gas species that vary significantly depending on nearby sources and sinks. These trace gasses include compounds such as CFCs/HCFCs which are particularly damaging to the ozone layer, and H2S which has a characteristic foul odor of rotten eggs and can be smelt in concentrations as low as 0.47 ppb. Some approximate amounts near the surface of some additional gasses are listed below. In addition to gasses, the atmosphere contains particles such as aerosol, which includes examples such as droplets, ice crystals, bacteria, and dust.
| Composition (ppt by volume unless otherwise stated) | ||
|---|---|---|
| Gas | Clean continental, Seinfeld & Pandis (2016)[3] | Simpson et al. (2010)[4] |
| Carbon monoxide, CO | 40-200 ppb p39 | 97 ppb |
| Nitric oxide, NO | 16 | |
| Ethane, C2H6 | 781 | |
| Propane, C3H8 | 200 | |
| Isoprene, C5H8 | 311 | |
| Benzene, C6H6 | 11 | |
| Methanol, CH3OH | 1967 | |
| Ethanol, C2H5OH | 75 | |
| Trichlorofluoromethane, CCl3F | 237 p41 | 252.7 |
| Dichlorodifluoromethane, CCl2F2 | 530 p41 | 532.3 |
| Chloromethane, CH3Cl | 503 | |
| Bromomethane, CH3Br | 9–10 p44 | 7.7 |
| Iodomethane, CH3I | 0.36 | |
| Carbonyl sulfide, OCS | 510 p26 | 413 |
| Sulfur dioxide, SO2 | 70–200 p26 | 102 |
| Hydrogen sulfide, H2S | 15–340 p26 | |
| Carbon disulfide, CS2 | 15–45 p26 | |
| Formaldehyde, H2CO | 9.1 ppb p37, polluted | |
| Acetylene, C2H2 | 8.6 ppb p37, polluted | |
| Ethene, C2H4 | 11.2 ppb p37, polluted | 20 |
| Sulfur hexafluoride, SF6 | 7.3 p41 | |
| Carbon tetrafluoride, CF4 | 79 p41 | |
| Total gaseous mercury, Hg | 0.209 p55 | |
History
[edit]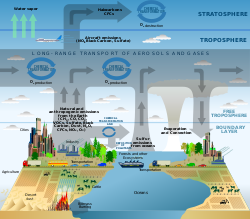
The first scientific studies of atmospheric composition began in the 18th century, as chemists such as Joseph Priestley, Antoine Lavoisier and Henry Cavendish made the first measurements of the composition of the atmosphere.[citation needed]
In the late 19th and early 20th centuries, researchers shifted their interest towards trace constituents with very low concentrations. One particularly important discovery for atmospheric chemistry was the discovery of ozone by Christian Friedrich Schönbein in 1840.
In the 20th century atmospheric science moved on from studying the composition of air to a consideration of how the concentrations of trace gasses in the atmosphere have changed over time and the chemical processes which create and destroy compounds in the air. Two particularly important examples of this were the explanation by Sydney Chapman and Gordon Dobson of how the ozone layer is created and maintained, and Arie Jan Haagen-Smit’s explanation of photochemical smog. Further studies on ozone issues led to the 1995 Nobel Prize in Chemistry award shared between Paul Crutzen, Mario Molina and Frank Sherwood Rowland.
In the 21st century the focus is now shifting again. Atmospheric chemistry is increasingly studied as one part of the Earth system. Instead of concentrating on atmospheric chemistry in isolation the focus is now on seeing it as one part of a single system with the rest of the atmosphere, biosphere and geosphere. An especially important driver for this link is chemistry and climate, such as how changing climate affects the recovery of the ozone hole and vice versa but also interaction of the composition of the atmosphere with the oceans and terrestrial ecosystems.[citation needed]
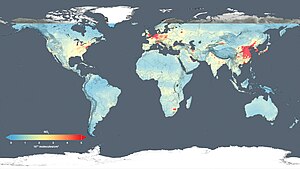
(NASA simulation; 9 November 2015)
Methodology
[edit]Observations, lab measurements, and modeling are the three central elements in atmospheric chemistry. Progress in atmospheric chemistry is often driven by the interactions between these components and they form an integrated whole. For example, observations may tell us that more of a chemical compound exists than previously thought possible. This will stimulate new modeling and laboratory studies which will increase our scientific understanding to a level where we can explain the observations.[citation needed][8]
Observation
[edit]Field observations of chemical systems are essential to understanding atmospheric processes and determining the accuracy of models. Atmospheric chemistry measurements can be long term to observe continuous trends or short term to observe smaller variations. Measurements can be made with observatories, satellites, and surface observations.
Routine observations of chemical composition show changes in atmospheric composition over time. Observatories such as the Mauna Loa and mobile platforms such as aircraft ships and balloons (e.g. the UK's Facility for Airborne Atmospheric Measurements) study chemical compositions and weather dynamics. An application of long term observations is the Keeling Curve - a series of measurements from 1958 to today which show a steady rise in the concentration of carbon dioxide (see also ongoing measurements of atmospheric CO2). Observations of atmospheric composition are increasingly made by satellites with important instruments such as GOME and MOPITT giving a global picture of air pollution and chemistry.
Surface observations have the advantage that they provide long term records at high time resolution but are limited in the vertical and horizontal space they provide observations from. Some surface based instruments e.g. LIDAR can provide concentration profiles of chemical compounds and aerosols but are still restricted in the horizontal region they can cover. Many observations are available online in Atmospheric Chemistry Observational Databases[citation needed][9]
Laboratory studies
[edit]Laboratory studies help understand the complex interactions from Earth’s systems that can be difficult to measure on a large scale. Experiments are performed in controlled environments, such as smog chambers, that allow for the individual evaluation of specific chemical reactions or the assessment of properties of a particular atmospheric constituent.[10] A closely related subdiscipline is atmospheric photochemistry, which quantifies the rate that molecules are split apart by sunlight, determines the resulting products, and obtains thermodynamic data such as Henry's law coefficients.
Laboratory measurements are essential to understanding the sources and sinks of pollutants and naturally occurring compounds. Types of analysis that are of interest include both those on gas-phase reactions, as well as heterogeneous reactions that are relevant to the formation and growth of aerosols. Commonly used analytical instruments include ambient and particulate air samplers, scanning mobility particle sizers, gas chromatographs, and mass spectrometers.
Modeling
[edit]Models are essential tools for interpreting observational data, testing hypotheses about chemical reactions, and predicting future concentrations of atmospheric chemicals. To synthesize and test theoretical understanding of atmospheric chemistry, researchers commonly use computer models, such as chemical transport models. These models can be seen as mathematical representations that replicate the behavior of the atmosphere. These numerical models solve the differential equations governing the concentrations of chemicals in the atmosphere.
Depending on the complexity, these models can range from simple to highly detailed. Models can be zero-, one-, two-, or three-dimensional, each with various uses and advantages. Three-dimensional chemical transport models offer the most realistic simulations but require substantial computational resources. These models can be global, simulating the atmospheric conditions across the Earth, or regional, focusing on specific areas with greater resolution. Global models typically have lower horizontal resolution and represent less complex chemical mechanisms but they simulate a larger area, while regional models can represent smaller areas with higher resolution and more detail.[11]
A major challenge in atmospheric modeling is balancing the number of chemical compounds and reactions included in the model with the accuracy of physical processes such as transport and mixing in the atmosphere. For example, box modeling is relatively simple and may include hundreds or even thousands of chemical reactions, but they typically use a very crude representation of atmospheric mixing. This makes them useful for studying specific chemical reactions, but limited in stimulating real-world dynamics. In contrast, 3D models are more complex, representing a variety of physical processes such as wind, convection, and atmospheric mixing. They also provide more realistic depictions of transportation and mixing. However, computational limits often simply chemical reactions and typically include fewer chemical reactions than box models. The trade-off between the two approaches lies in resolution and complexity.
To simplify the creation of these complex models, some researchers use automatic code generators like Autochem or Kinetic PreProcessor. These tools help automate the model-building process by selecting relevant chemical reactions from databases based on a user-defined set of chemical constituents. Once the reactions are chosen, the code generator automatically constructs the ordinary differential equations that describe their time evolution, greatly reducing the time and effort required for model construction.
One important current trend is using atmospheric chemistry as part of Earth system models. These models integrate atmospheric chemistry with other Earth system components, such as biosphere and hydrosphere, enabling the study of complex interactions between climate, atmospheric composition, and ecosystems.
See also
[edit]- Oxygen cycle
- Ozone-oxygen cycle
- Paleoclimatology
- Scientific Assessment of Ozone Depletion
- Tropospheric ozone depletion events
References
[edit]- ^ Cairns, Iver (23 September 1999). "Earth's Atmosphere". The University of Sydney. Retrieved 7 April 2021.
- ^ Zimmer, Carl (3 October 2013). "Earth's Oxygen: A Mystery Easy to Take for Granted". The New York Times. Retrieved 3 October 2013.
- ^ Seinfeld, John; Pandis, Spyros (2016). Atmospheric Chemistry and Physics - from Air Pollution to Climate Change, 3rd ed. Hoboken, New Jersey: Wiley. ISBN 9781119221173.
- ^ Simpson, I. J.; Blake, N. J.; Barletta, B.; Diskin, G. S.; Fuelberg, H. E.; Gorham, K.; Huey, L. G.; Meinardi, S.; Rowland, F. S.; Vay, S. A.; Weinheimer, A. J.; Yang, M.; Blake, D. R. (2010). "Characterization of trace gases measured over Alberta oil sands mining operations: 76 speciated C2–C10 volatile organic compounds (VOCs), CO2, CH4, CO, NO, NO2, NO, O3 and SO2". Atmospheric Chemistry and Physics. 10 (23): 11931–11954. Bibcode:2010ACP....1011931S. doi:10.5194/acp-10-11931-2010. ISSN 1680-7324. S2CID 62782723.
- ^ St. Fleur, Nicholas (10 November 2015). "Atmospheric Greenhouse Gas Levels Hit Record, Report Says". The New York Times. Retrieved 11 November 2015.
- ^ Ritter, Karl (9 November 2015). "UK: In 1st, global temps average could be 1 degree C higher". AP News. Retrieved 11 November 2015.
- ^ Cole, Steve; Gray, Ellen (14 December 2015). "New NASA Satellite Maps Show Human Fingerprint on Global Air Quality". NASA. Retrieved 14 December 2015.
- ^ Brasseur, Guy; Prinn, Ronald; Pszenny, Alexander (2003). Atmospheric Chemistry in a Changing World. New York: Springer-Verlag BerIin Heidelberg. ISBN 978-3-642-62396-7.
- ^ "Air Quality Modeling - Surface and Upper Air Databases". U.S. Environmental Protection Agency. March 19, 2024.
- ^ National Academies of Sciences, Engineering, and Medicine (2016). Future of Atmospheric Research: Remembering Yesterday, Understanding Today, Anticipating Tomorrow. Washington, DC: The National Academies Press. p. 15. ISBN 978-0-309-44565-8.
- ^ Brasseur, Guy P.; Orlando, John J.; Tyndall, Geoffrey S. (1999). Atmospheric Chemistry and Global Change. United States: The National Academies Press. pp. 439–441. ISBN 0-19-510521-4.
Further reading
[edit]- Finlayson-Pitts, Barbara J.; Pitts, James N., Jr. (2000). Chemistry of the Upper and Lower Atmosphere. Academic Press. ISBN 0-12-257060-X.
- Iribarne, J. V. Cho, H. R. (1980). Atmospheric Physics, D. Reidel Publishing Company.
- Seinfeld, John H.; Pandis, Spyros N. (2006). Atmospheric Chemistry and Physics: From Air Pollution to Climate Change (2nd Ed.). John Wiley and Sons, Inc. ISBN 0-471-82857-2.
- Warneck, Peter (2000). Chemistry of the Natural Atmosphere (2nd Ed.). Academic Press. ISBN 0-12-735632-0.
- Wayne, Richard P. (2000). Chemistry of Atmospheres (3rd Ed.). Oxford University Press. ISBN 0-19-850375-X.
External links
[edit]- WMO Scientific Assessment of Ozone Depletion: 2006
- IGAC The International Global Atmospheric Chemistry Project
- Paul Crutzen Interview - freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone, talking to Nobel Laureate Harry Kroto, the Vega Science Trust
- The Cambridge Atmospheric Chemistry Database is a large constituent observational database in a common format.
- Environmental Science Published for Everybody Round the Earth
- NASA-JPL Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies
- Kinetic and photochemical data evaluated by the IUPAC Subcommittee for Gas Kinetic Data Evaluation
- Atmospheric Chemistry Glossary at Sam Houston State University
- Tropospheric chemistry
- Calculators for use in atmospheric chemistry Archived 2010-12-09 at the Wayback Machine
- An illustrated elementary assessment of the composition of air